Description
PCBA For the Medical Industry
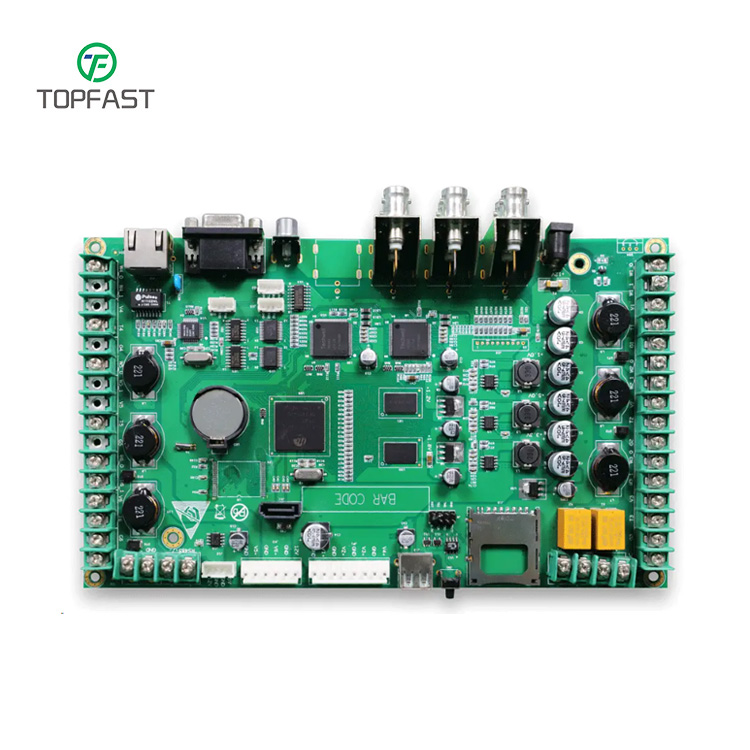
PCBA (Printed Circuit Board Assembly) is a process that involves mounting electronic components onto a printed circuit board (PCB) and soldering them in place to create a functional electronic device. In the medical industry, PCBA is used to manufacture a wide range of medical devices, including diagnostic equipment, monitoring devices, and surgical instruments.
Medical devices that use PCBA are typically required to meet strict regulatory standards to ensure their safety and effectiveness. As a result, the PCBA process for medical devices must be carefully controlled to ensure that the finished product meets these standards. This may involve the use of specialized materials, such as biocompatible plastics and metals, and the implementation of strict quality control measures.
The use of PCBA in the medical industry allows for the creation of highly precise and reliable devices that are essential for the diagnosis, treatment, and monitoring of patients. It also enables the development of new and innovative medical technologies that can improve patient outcomes and quality of care. If you are interested in our PCB Assembly, pls feel free to contact us.
Advantages
Here are several advantages of using PCBA in the medical industry:
1. Precision: PCBA allows for the creation of highly precise and accurate medical devices, which is essential for many diagnostic and monitoring applications.
2. Reliability: The PCBA process is designed to create durable and reliable medical devices that can withstand the demands of the healthcare environment.
3. Customization: PCBA can be customized to meet the specific needs and requirements of different medical applications. This allows manufacturers to create devices that are tailored to the unique needs of the healthcare industry.
4. Cost-effectiveness: The use of PCBA can help to reduce the overall cost of medical device manufacturing by allowing for the use of automated processes and standard components.
5. Speed of production: The PCBA process can be completed relatively quickly, allowing manufacturers to meet the high demand for medical devices on time.
Improved patient care: By creating reliable and precise medical devices, PCBA helps to improve patient care and outcomes. This can lead to better patient satisfaction and overall healthcare quality.
Core Elements of Quality Systems
-
Triple Certification System
-
Process Control Highlights
Key Technical Challenges & Solutions
| Challenge Type |
Technical Solution |
Performance Metrics |
| Sterilization Resistance |
Special encapsulation materials |
Withstands 200 autoclave cycles |
| EMI Control |
Multilayer shielding structures |
60dB radiation reduction |
| Long-term Reliability |
Gold bonding process |
MTBF >100,000 hours |
| Biocompatibility |
Parylene coating |
Complies with ISO 10993-5 |
Performance Requirements for Typical Applications
-
Implantable Devices
-
Imaging Equipment
-
Emergency Devices
7 Key Criteria for Supplier Selection
-
Class III medical device production certification
-
Medical-grade component supply chain
-
Cleanroom area ≥1000㎡
-
Failure analysis lab configuration
-
Product change control system
-
Long-term aging testing capability
-
Clinical collaboration experience
-
Process Capabilities
✓ 01005 component placement
✓ 50μm fine-pitch BGA
✓ Rigid-flex board processes
-
Inspection Systems
✓ 100% flying probe test coverage
✓ 3D X-ray inspection
✓ Ionic contamination testing
-
Special Processes
✓ Biocompatible coatings
✓ Sterile packaging
✓ Conformal coating
Implementation Recommendations
-
Conduct FMEA analysis during the design phase
-
Establish reliability growth models
-
Implement V&V verification systems
-
Improve change management processes
Future Outlook: With advancements in brain-computer interfaces and nanorobotics, medical PCBs will evolve toward innovative directions like neural electrode arrays and biodegradable circuits, demanding higher assembly precision. Manufacturers are advised to invest in molecular-level packaging and self-healing circuit technologies.