Product Details
Medical Equipment PCBA refers to the printed circuit board assembly specifically designed and manufactured for use in medical equipment. It is the integration of electronic components onto a PCB that forms a crucial part of medical devices used for diagnostics, treatment, monitoring, or other medical applications.
Medical Equipment PCBA encompasses a wide range of devices, including but not limited to:
Imaging Systems: This category includes devices such as X-ray machines, MRI (Magnetic Resonance Imaging) scanners, CT (Computed Tomography) scanners, ultrasound machines, and other diagnostic imaging equipment.
The PCBA in these systems may involve complex analog and digital circuitry, specialized image processing ICs, high-speed data transmission interfaces, and advanced sensor technologies.
Patient Monitoring Devices: These devices are used to measure and monitor vital signs, such as heart rate, blood pressure, oxygen saturation, and more. Examples include ECG (Electrocardiogram) monitors, pulse oximeters,
blood pressure monitors, and multi-parameter patient monitors. The PCBA for patient monitoring devices typically incorporates sensors, amplifiers, filters, microcontrollers, and display interfaces.
Surgical Instruments: Medical PCBA can be found in various surgical instruments and tools used during surgical procedures. These may include electrosurgical units, laser surgical devices, robotic surgical systems, and other
electronically controlled instruments. In such PCBA, precision control circuits, motor drivers, position sensors, and communication interfaces may be present.
Infusion Pumps and Drug Delivery Systems: These devices are used for administering medications, fluids, or nutrients to patients. The PCBA in infusion pumps and drug delivery systems may involve flow sensors, motor controls,user interface components, wireless connectivity options, and safety features like alarms and fault detection.
Laboratory Equipment: Medical PCBA can also be found in laboratory equipment used for medical research, diagnostics, or analysis. This includes devices such as PCR (Polymerase Chain Reaction) machines, centrifuges,spectrophotometers, and other specialized laboratory instruments. The PCBA in these devices may include temperature control circuits, precision measurement components, data acquisition systems, and interfaces for external communication.
Therapeutic Devices: This category includes medical devices used for therapeutic purposes, such as electrotherapy devices, physical therapy equipment, or respiratory support systems. The PCBA in these devices may involve control circuits, motor drives, sensors, and interfaces for adjusting therapy settings and monitoring patient responses.
Medical Equipment PCBA is designed with strict adherence to regulatory standards, safety requirements, and reliability considerations. The assembly process involves careful component selection, precise soldering techniques, rigorous testing, and validation to ensure the performance, accuracy, and safety of the medical equipment.
For a PCB manufacturer involved in medical equipment PCB assembly, several crucial steps and considerations must be addressed to ensure the production of high-quality and reliable PCBAs. Here are some key actions they should undertake:
Compliance with Regulatory Standards: The manufacturer must have a thorough understanding of relevant regulatory standards and guidelines specific to the medical industry, such as ISO 13485, FDA (Food and Drug Administration) regulations, or other regional requirements. Compliance with these standards is essential to ensure the safety and reliability of the medical equipment.
Design for Manufacturability (DFM): Collaboration between the PCB designer and the manufacturer is crucial. A DFM review should be conducted to ensure that the design is optimized for efficient manufacturing processes, considering factors such as component placement, signal integrity, thermal management, and manufacturability constraints.
Material Selection: Choosing appropriate materials for medical equipment PCBAs is critical. The manufacturer should work closely with the designers and customers to select suitable PCB substrates, solder masks, surface finishes, and other materials that meet the specific requirements of the medical device, including biocompatibility, sterilizability, thermal performance, and electrical properties.
Component Procurement and Verification: The manufacturer should establish strong relationships with trusted suppliers to source components that meet the required quality and reliability standards. It is essential to verify the authenticity and traceability of components, ensuring they are procured from reputable sources and comply with necessary regulations.
Control of Contamination: Ensuring cleanliness and controlling contamination during the PCBA process are crucial for medical equipment. Implementing proper ESD (Electrostatic Discharge) precautions, cleanroom environments, and adherence to appropriate cleaning and handling procedures are important to prevent any adverse effects on the PCBs and associated components.
Strict Quality Control: Rigorous quality control measures should be implemented throughout the manufacturing process. This includes comprehensive inspection and testing, both automated and manual, to verify component placement accuracy, solder joint integrity, electrical functionality, and adherence to design specifications. Statistical Process Control (SPC) techniques can be utilized to monitor and maintain consistent quality during production.
Traceability and Documentation: Maintaining comprehensive documentation and implementing robust traceability systems are crucial for medical equipment PCBAs. The manufacturer should track and record critical information related to components, manufacturing processes, testing results, and any necessary certifications or compliance documentation.
Collaboration with Customers: Effective communication and collaboration with the customer throughout the entire manufacturing process is essential. This includes regular updates, addressing design concerns, discussing specific requirements, and ensuring that the final PCBAs meet the customer's expectations and regulatory standards.
By the above steps and adhering to industry best practices, a PCB manufacturer can ensure the production of high-quality and reliable PCBAs for medical equipment while meeting the demanding regulatory requirements of the medical industry.
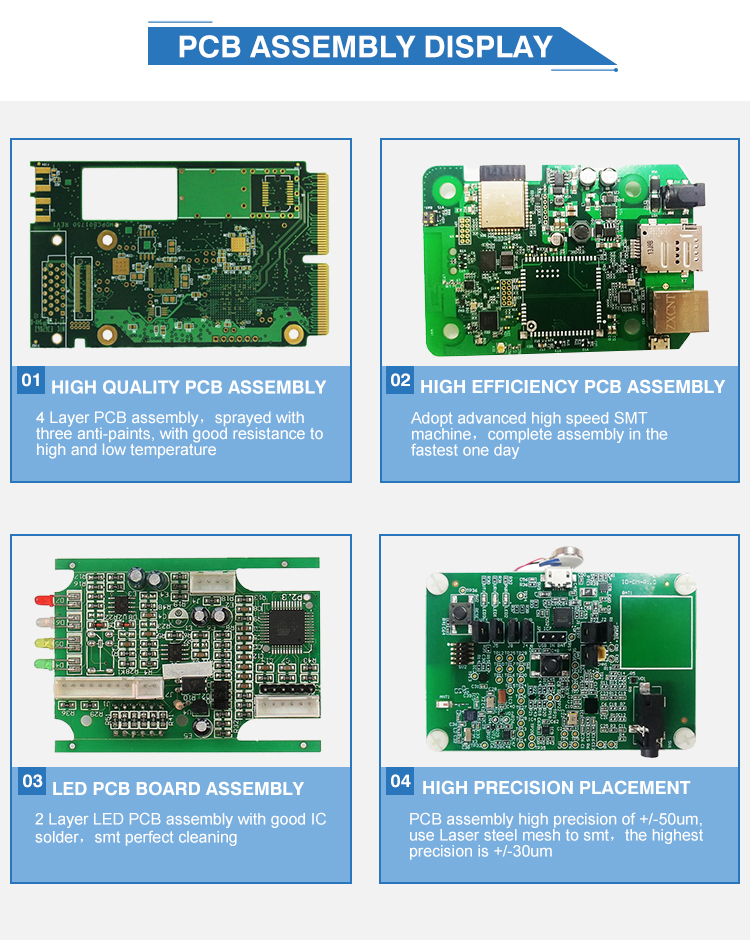
Topfast is a professional PCB & PCBA manufacturer in Shenzhen China.We provide high quality bare PCB and PCB assembly service,including components sourcing, function test,conformal coating and complete assembly for clients all over the world.
We provides superior service for automotive electronics, medical electronics, telecommunications, industrial control, smart home and other industries around the world.

Address of Plant
PCB Factory:
A1 Building, B Zone, Ditang Industrial Zone, Ditang Road, Shajing Street, Bao'an District, Shenzhen, China
PCBA Factory:
Room 805, Room 806, Room 809, No. 96, Chuangqiang Road, Ningxi Street, Zengcheng District, Guangzhou City, Guangdong Province, P.R. China
Office Address:
Room 805, Room 806, Room 809, No. 96, Chuangqiang Road, Ningxi Street, Zengcheng District, Guangzhou City, Guangdong Province, P.R. China